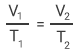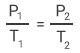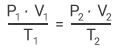The Gas Laws
Boyle's Gas Law
The gas laws came about as a result of various attempts to establish the relationship of the volume and pressure of a gas at a constant temperature. The one that most people have heard about is Boyle’s Law. Robert Boyle was born in Ireland in 1627, the 14th child of the Earl of Cork. Robert was a pioneer of modern chemistry. He was also a founding member of the Royal Society.
In 1662, he published his second edition of his controversial scientific work, “New Experiments Physico-Mechanicall, Touching the Spring of Air, and its Effects”. This second edition never explicitly stated Boyle’s law. The appendix contained a table of numbers that showed the inverse relationship between pressure and the volume of air.
Mariotte's Gas Law.
In 1672 the French priest, Edme Mariotte, also independently published his results of the inverse relationship between volume and pressure. This relationship carried out at a constant temperature generally became known in France as Mariotte’s law.
Charles’s Gas Law
Jacques Alexandre César Charles, was a French physicist and mathematician also known for his work with gases and hydrogen balloon flights. His experiments with oxygen, hydrogen, and carbon dioxide in 1787 demonstrated that all these gas volumes increased identically with higher temperatures. This occurred when the pressure remained constant.
Gay-Lussac’s Gas Law
The French chemist, Joseph Louis Gay-Lussac, made several daring ascents in hydrogen balloons. These assents were over 7,000 metres above sea level. During the ascents he also recorded pressure, temperature, and humidity measurements. From his own and others experiments he was able to deduce in 1808 that for an ideal gas of given mass and constant volume, the pressure is directly proportional to its absolute temperature.
The Combined Gas Law
The combined gas law uses the three laws discussed above. These show the relationship between the pressure, volume and temperature for a fixed mass of gas.
That’s the history and science bit, but how can this be used practically.
There are many situations when engineers and scientists need to know the relationship between a gas volume, pressure, and temperature. Visit my page on Hydro-Pneumatic Accumulators to see a practical application.